We accept NHS purchase orders

INNOVA SARS-CoV-2 Rapid Antigen Lateral Flow Qualitative Test Kit. Box of 25 kits.
If you are looking to place an order of significant quantity for this item, we may be able to help you with a bespoke quote.
Please complete all the details required below.
Same day delivery is available for London and the South East. Please email sales@primarycaresupplies.co.uk for a quote.
Product Demonstration Video
Product Demonstration Video for Healthcare Professionals
-
The Innova SARS-CoV-2 COVID Lateral Flow Rapid Antigen test kit facilitates the detection of COVID-19 with rapid return of results.
-
The Innova SARS-CoV-2 COVID Antigen Test has been tested by the UK Government. Click Here for details.
-
This antigen test is intended for use by clinical professionals.
The Rapid COVID-19 Innova Antigen Test is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nose and/or throat swab from individuals who are suspected of COVID-19 by their healthcare provider.
It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections.
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. Currently, individuals infected by SARS-CoV-2 are the main source of transmission; asymptomatic carriers are also known as a source of transmission. Based on the current epidemiological information, the incubation period can last from 1 to 14 days, where the average time before symptoms occur is about 5 days. Symptoms include fever or chills, cough, shortness of breath, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, runny nose, nausea or vomiting and diarrhea.
These rapid lateral flow tests generate accurate results, usually within 15 minutes. This Antigen test has 100% specificity, 98.98% overall accuracy.
-
Qualitative, visually read results in 15 minutes
-
No instrument required
-
Room temperature storage or refrigerated (2-30⁰C / 36-86⁰F)
-
Procedural internal control included
-
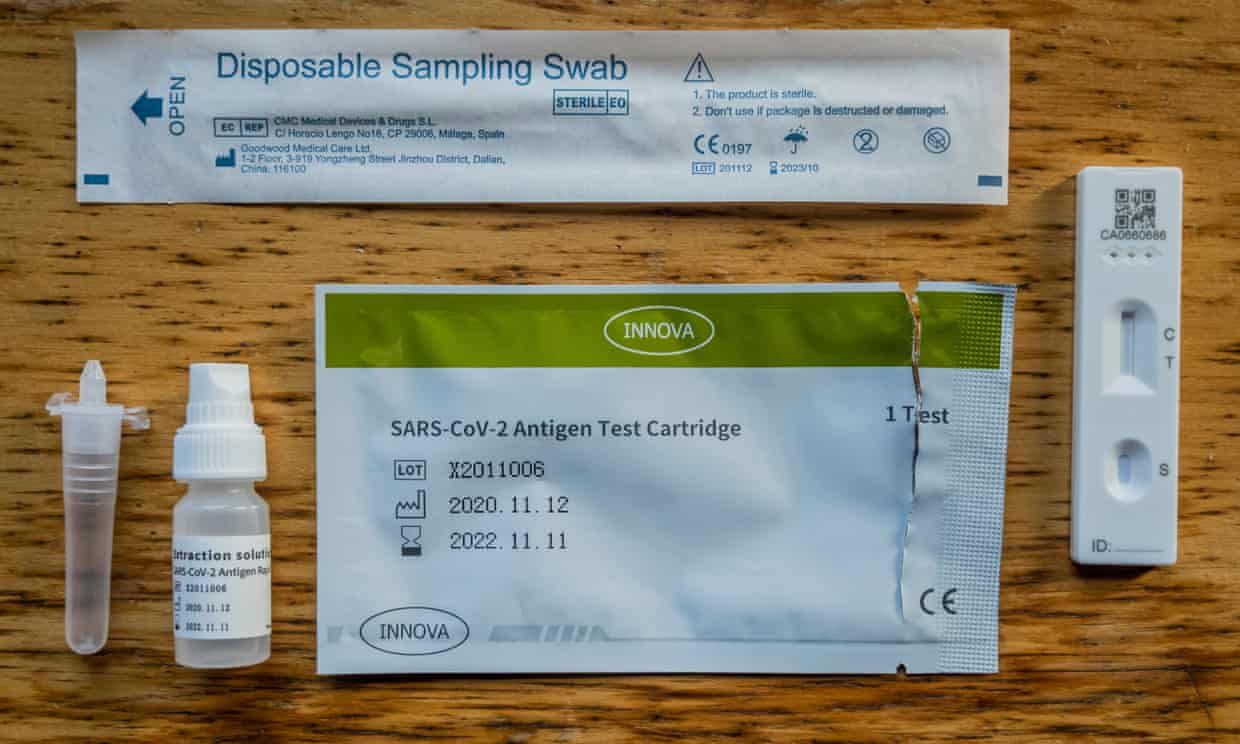
Sterile Swabs, Extraction Buffer and Extraction Tubes included
-
Dispose of used test components according to the IFU
This item line is exempt from our usual returns policy. This item is non-returnable or exchangeable.
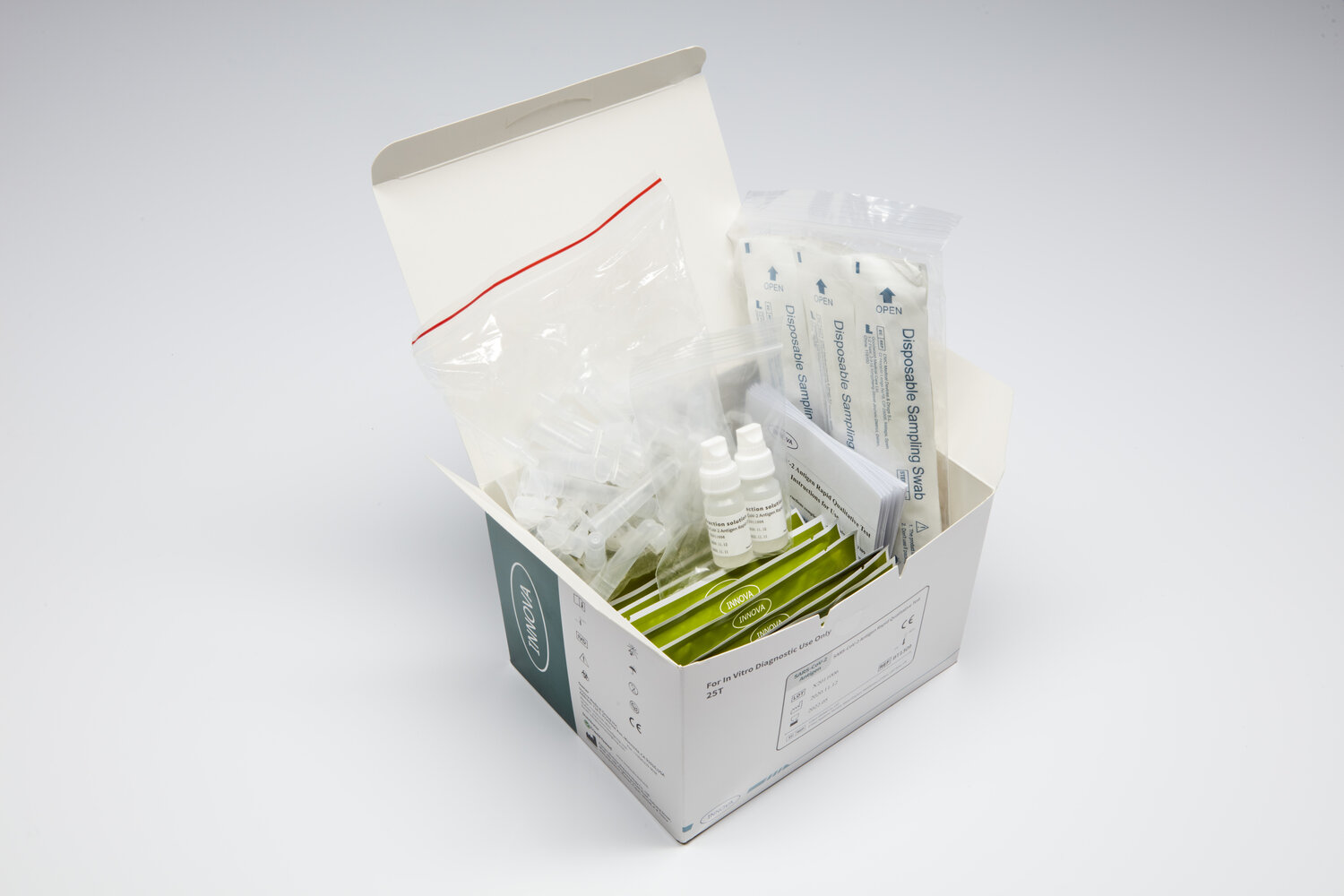
Box Content:
Each box contains everything to perform 25 tests, and comprises of:
-
Test device – individual foil wrapped with desiccant x 25 cassettes
-
Extraction buffer solution x 2 bottles
-
Sterile swabs x 25
-
Extration tubes x 25
-
Instructions for use and Quick Reference Guide
Test Procedure:
1a. Specimen Collection (Nasal Swab Collection)
-
Use the swab supplied in the kit
-
Carefully insert the swab into one nostril.
-
Swab tip should be inserted about 2.5 cm (1 inch).
-
Roll swab firmlyagainst thenostrilinside wallfirmly 5 times
-
Withdraw the swab from the nasal cavity.
-
Swab both nostrils

1b. Specimen Collection (Throat Swab Collection)
-
Use the swab supplied in the kit
-
Open your mouth wide and rub the fabric tip over both tonsils (or where they would have been)
-
Do this firmly 3 times
-
Roll swab firmlyagainst thenostrilinside wallfirmly 5 times
-
Carefully withdraw the swab from throat

2. Sample preparation
-
Insert the test extraction tube into the workstation
-
Make sure that the tube is standing firm and reaches the bottom of the workstation
-
Add 0.3 mL (about 6 drops) of the sample extraction buffer into the extraction tube.
-
Insert the swab into the extraction tube which contains 0.3 mL of the extraction buffer. Roll the swab at least 10 seconds while pressing the head against the bottom and side of the extraction tube. Leave the swab in the extraction tube for 1 minute.
-
Squeeze the tube several times with fingers from outside of the to immerse the swab
-
Remove the swab. The extracted solution will be used as test sample.
-
Fit the cap tip with filter on top the extraction tube tightly






3. Interpretation of Results
-
Allow the test device, test sample and buffer to equilibrate to room temperature prior to testing
-
Remove test device from the sealed pouch just prior to the testing and lay flat on work bench.
-
Ensure the nozzle with filter is fitted on to the sample extraction tube tightly.
-
Reverse the sample extraction tube, and add 2 drops ( of test sample by squeezing the extracted solution tube into the sample window.
-
Wait for the colored band(s) to appear. The result should be read in 20 minutes.
-
Do not interpret the result after 30 minutes.
Test results:
-
POSITIVE - The presence of two lines as control line (C) and test line (T) within the result window indicates a positive result.

-
NEGATIVE* - The presence of only control line (C) within the result window indicates a negative result.

-
INVALID - If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. It is recommended that the specimen be re-tested using a new test.

Note: The intensity of color in the test line region (T) may vary depending on the concentration of analytes present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive. Please note that this is a qualitative test only and cannot determine the concentration of analytes in the specimen. Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
(*Negative results do not rule out SARS-CoV-2 infection, particularly those who have been in contact with the virus. Follow up testing with a molecular diagnostic test should be considered to rule out infection in these individuals.)
In-vitro use only. Please note, each box includes 1 x work station and 2x buffer solutions, therefore it can only be split in 2 packs of 10 test kits each.
For further information please refer to FAQ tab of this product page.
Stock items are usually dispatched and delivered within 1 to 3 working days.
In most cases, items in stock that are ordered before 12 noon are delivered on a next working day service.
For accurate delivery information, please call 0845 862 9500 or press the live chat button to speak to one of our online sales team.
If you need this item urgently please contact us using the live chat or call us on 0845 862 9500 to enquire about our premium rate delivery service including same-day, timed and weekend deliveries.
For more information about delivery, please visit our delivery information page here
What is antigen testing?
Antigen tests detect proteins specific to a virus that appears in infected individuals, which indicates active infection. Rapid antigen testing is beneficial because of their portability, ease-of-use, and rapid time to result.
These tests are designed to detect a live virus in people who are highly infectious, whether they are displaying symptoms or not. Their rapid turnaround time allows for mass testing in workplaces and schools without the need for expensive laboratory equipment and analysis.
What Is An Antigen Lateral Flow Test?
Lateral flow antigen tests work by taking a sample from the nose or back of the throat and testing that sample for the presence of antigens, the signature proteins of the virus. They show results visually, in the same way as many pregnancy tests.
How Quickly Does the Innova Rapid COVID-19 Antigen Test Deliver a Result?
An Antigen lateral flow test kits provide a visual, easy to read result in as little as 20 minutes, which means those who test positive can be instructed to isolate quickly and minimise exposure of COVID-19 in your school, workplace or community.
A positive test should be confirmed by a governament approved CPR test.
What is the Difference Between an Antibody and an Antigen Test?
An antibody test is a blood test to determine if you’ve already been exposed to COVID-19 and have developed the relevan antibodies.
It works by detecting antibodies to SARS COV-2 infection in your blood or serum.
A lateral flow (LFT) antigen test looks for an active COVID-19 infection such as the SARS-CoV-2 virus. If your antigen test is positive, you should self-isolate at home and follow government guidance.
Does the Innova Antigen Testing Kit Contain Everything I Need To Perform The Test?
The Innova Rapid Lateral Flow COVID-19 testing kits come supplied with all the necessary tools to carry out the test - no extra equipment is needed. The packs include a workstation to lay out the contents in a way that helps workplaces deliver mass testing with ease, organisation and efficiency.
Clear instructions are supplied on how to perform the test and read the results.
While no specialist equipment or laboratory analysis is required, we recommend that these products are used by healthcare professional or trained and competent people.
Are Specialist Personnel Required to Administer the LFT?
These LFD antigen tests are designed to be used by Healthcarte professionals or by trained and competent individuals. The Innova lateral flow test is currently used by the governament for home testing of key workers.
How Often Should I Check for Symptoms or Test?
As an employer, you need to decide the appropriate timescale between tests. For example, in some sectors (such as health and social care) where interactions with vulnerable individuals are common, repeat rapid antigen testing may be required more often.
This depends on the safety measures that your organisation needs to put in place. The scale of these will be impacted by the size of your workforce, the risk factors identified in your contingency plan and the effect of any requirement to isolate on your ability to continue running your business.
A positive test on the the Healgen Rapid Antigen test cassette will require for the individual to self isolate and book a Governament approved PCR Covid test immediately for confirmation.
How Do I Store The Innova Antigen Test Kit?
The Innova Rapid COVID-19 Antigen Test kits can be stored at room temperature or refrigerated (2-30°C) - please follow instructions on each pack. The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.
What is the shelf-life of the Healgen Lateral Flow Test?
Normally the Healgen Antigen Test have at least 18 months shelf-life, some batches do extend to 24 months expiry date.
What is the extraction buffer?
The extraction buffer includes components to dissociate viral proteins from their surfaces to be used as a target for the test. The method of the extraction buffer is used to prepare the test sample by dissolving the virus shell, which also deactivates the virus.
Does the test kit include transport media?
The test kit includes an extraction buffer that is used to prepare the specimen for testing purposes. The extraction buffer IS NOT transport media and should not be used to preserve or transport specimens.
Can the test be used with alternative specimen types such as nasal swabs or any specimen contained in viral transport media?
The Innova Sars-CoV-2 Rapid Antigen Test is for direct nasal or/and throat swab specimen only. The test can only be used with the swab provided in the kit.
Can I collect the specimen and test at a later time?
The specimen should be tested immediately after collection. The specimen can be retained up to 1 hour following collection if immediate testing of specimens is not possible. Dispose of the specimen and recollect if retained for more than 1 hour.
Can I freeze the device for long-term storage?
The device should never be frozen. If refrigerated, allow the test kit components to reach room temperature before use.
How do I safetely dispose of a used Innova lateral Flow Antigen Test kit?
According to the governament instruction leaflets to Carehomes for safe disposal od lateral flow antigen test kits, these can be bagged and disposed of in the normal household waste. Care Home LFD Self test Guidance
Could vaccination cause a false-positive COVID-19 lateral flow device (LFD) antigen test result?
The Innova Antigen LFD test detects a different protein of the virus than the one encoded in the vaccine. There is no possibility of cross reaction.
Would the Innova Antigen lateral flow test kit detect the new variants of the virus?
Yes, the governament has tested the Innova rapid antigen test for the new VUI-202012/01 Covid-19 variant.
Lateral Flow Test Evaluation of VUI 20201201 link
INNOVA SARS-CoV-2 Rapid Antigen Test characteristics
- Assay format: Lateral flow test / immunochromatographic
- Instrument: No
- Testing time: 20-30 minutes
- Specificity: 100%
- Overall Acuuracy: 98.98%
- Antigen: N
- Sample material: Nasal and/or throat swab
Performance Characteristics
- The INNOVA SARS-CoV-2 Covid-19 Antigen Test (Swab) has been evaluated with specimens obtained from patients.
- A commercialised molecular assay was used as the reference method.
- The results show that the Innova Covid-19 Antigen Test (Swab) has a high overall relative accuracy.
Validation and Assessment
Tested by Public Health England & validated by the UK government
- Rapid diagnosis of SARS-CoV-2 infections
- Evaluated and Validated by the UK Government: 1 of only 3 tests for use by schools, hospitals, and the army. Used by the NHS in the UK. More details HERE.
- This test is being rolled out by the UK government to mass test the population in Liverpool and other cities, See www.gov.uk for more information (test 1 on the list).
- CE Marked: For professional use
- ISO13485: Innova manufactures in accordance with their ISO13485 Quality Management accreditation.

INNOVA SARS-CoV-2 Rapid Antigen Lateral Flow Qualitative Test Kit. Box of 25 kits.
SKU: DRA-INNOVA
Price:
Description
Specification ss
https://www.portableoxygen.co.uk/intermedicaladmin/cms/wysiwyg/directive/___directive/e3ttZWRpYSB1cmw9IjxoMz7CoDwvaDM-DQo8aDM-PHN0cm9uZyBzdHlsZT0iZm9udC1zaXplOiAxNHB4OyI-SU5OT1ZBIFNBUlMtQ29WLTIgUmFwaWQgQW50aWdlbiBUZXN0IGNoYXJhY3RlcmlzdGljczwvc3Ryb25nPjwvaDM-DQo8ZGl2IGNsYXNzPSJzcGVjaWZpY2F0aW9uIj4NCjxkaXYgY2xhc3M9InN5c3RlbXNwZWNpZmljYXRpb24gc2VjdGlvbiI-DQo8c2VjdGlvbiBjbGFzcz0iYy1wZHAtc3BlY3MtY29udGFpbmVyIHgtY29tcG9uZW50LXNwYWNpbmcganMtc3BlY2lmaWNhdGlvbnMtY29udGFpbmVyIGpzLXNwZWNpZmljYXRpb25zLWNvbnRhaW5lci1hdXRob3IgeC1vdXRlci1tYXJnaW5zIiBkYXRhLW1vZHVsZT0ic3lzdGVtc3BlY2lmaWNhdGlvbiI-DQo8ZGl2IGNsYXNzPSJjLXBkcC1zcGVjLXdyYXBwZXIiPg0KPHVsPg0KPGxpIGNsYXNzPSJjLXBkcC1zcGVjIj48c3BhbiBjbGFzcz0iYy1wZHAtc3BlYy1sYWJlbCI-QXNzYXkgZm9ybWF0OsKgPC9zcGFuPkxhdGVyYWwgZmxvdyB0ZXN0IC8gaW1tdW5vY2hyb21hdG9ncmFwaGljPC9saT4NCjxsaSBjbGFzcz0iYy1wZHAtc3BlYyI-PHNwYW4gY2xhc3M9ImMtcGRwLXNwZWMtbGFiZWwiPkluc3RydW1lbnQ6wqA8L3NwYW4-Tm88L2xpPg0KPGxpIGNsYXNzPSJjLXBkcC1zcGVjIj48c3BhbiBjbGFzcz0iYy1wZHAtc3BlYy1sYWJlbCI-VGVzdGluZyB0aW1lOiAyMDwvc3Bhbj4tMzAgbWludXRlczwvbGk-DQo8bGkgY2xhc3M9ImMtcGRwLXNwZWMiPjxzcGFuIGNsYXNzPSJjLXBkcC1zcGVjLWxhYmVsIj5TcGVjaWZpY2l0eTrCoDwvc3Bhbj4xMDAlPC9saT4NCjxsaSBjbGFzcz0iYy1wZHAtc3BlYyI-PHNwYW4gY2xhc3M9ImMtcGRwLXNwZWMtbGFiZWwiPk92ZXJhbGwgQWN1dXJhY3k6IDwvc3Bhbj45OC45OCXCoDwvbGk-DQo8bGkgY2xhc3M9ImMtcGRwLXNwZWMiPjxzcGFuIGNsYXNzPSJjLXBkcC1zcGVjLWxhYmVsIj5BbnRpZ2VuOsKgPC9zcGFuPk48L2xpPg0KPGxpIGNsYXNzPSJjLXBkcC1zcGVjIj48c3BhbiBjbGFzcz0iYy1wZHAtc3BlYy1sYWJlbCI-U2FtcGxlIG1hdGVyaWFsOsKgPC9zcGFuPk5hc2FsIGFuZC9vciB0aHJvYXQgc3dhYjwvbGk-DQo8L3VsPg0KPHA-PHN0cm9uZz5QZXJmb3JtYW5jZSBDaGFyYWN0ZXJpc3RpY3M8L3N0cm9uZz48L3A-DQo8dWw-DQo8bGk-VGhlIElOTk9WQSBTQVJTLUNvVi0ywqBDb3ZpZC0xOSBBbnRpZ2VuIFRlc3QgKFN3YWIpIGhhcyBiZWVuIGV2YWx1YXRlZCB3aXRoIHNwZWNpbWVucyBvYnRhaW5lZCBmcm9tIHBhdGllbnRzLjwvbGk-DQo8bGk-QSBjb21tZXJjaWFsaXNlZCBtb2xlY3VsYXIgYXNzYXkgd2FzIHVzZWQgYXMgdGhlIHJlZmVyZW5jZSBtZXRob2QuPC9saT4NCjxsaT5UaGUgcmVzdWx0cyBzaG93IHRoYXQgdGhlIElubm92YcKgQ292aWQtMTkgQW50aWdlbiBUZXN0IChTd2FiKSBoYXMgYSBoaWdoIG92ZXJhbGwgcmVsYXRpdmUgYWNjdXJhY3kuPC9saT4NCjwvdWw-DQo8cD48c3Ryb25nPlZhbGlkYXRpb24gYW5kIEFzc2Vzc21lbnQ8L3N0cm9uZz48L3A-DQo8cD7CoDwvcD4NCjxwPjxzdHJvbmc-VGVzdGVkIGJ5IFB1YmxpYyBIZWFsdGggRW5nbGFuZCAmYW1wOyB2YWxpZGF0ZWQgYnkgdGhlIFVLIGdvdmVybm1lbnQ8L3N0cm9uZz48L3A-DQo8dWw-DQo8bGk-UmFwaWQgZGlhZ25vc2lzIG9mIFNBUlMtQ29WLTIgaW5mZWN0aW9uczwvbGk-DQo8bGk-PHN0cm9uZz5FdmFsdWF0ZWQgYW5kIFZhbGlkYXRlZCBieSB0aGUgVUsgR292ZXJubWVudDo8L3N0cm9uZz4gMSBvZiBvbmx5IDMgdGVzdHMgZm9yIHVzZSBieSBzY2hvb2xzLCBob3NwaXRhbHMsIGFuZCB0aGUgYXJteS4gVXNlZCBieSB0aGUgTkhTIGluIHRoZSBVSy4gTW9yZSBkZXRhaWxzwqA8YSBocmVmPSJodHRwczovL3d3dy5nb3YudWsvZ292ZXJubWVudC9wdWJsaWNhdGlvbnMvYXNzZXNzbWVudC1hbmQtcHJvY3VyZW1lbnQtb2YtY29yb25hdmlydXMtY292aWQtMTktdGVzdHMvbGF0ZXJhbC1mbG93LWRldmljZXMtcmVzdWx0cyIgdGFyZ2V0PSJfYmxhbmsiIHJlbD0ibm9vcGVuZXIgbm9yZWZlcnJlciI-SEVSRTwvYT4uPC9saT4NCjxsaT5UaGlzIHRlc3QgaXMgYmVpbmcgcm9sbGVkIG91dCBieSB0aGUgVUsgZ292ZXJubWVudCB0byBtYXNzIHRlc3QgdGhlIHBvcHVsYXRpb24gaW4gTGl2ZXJwb29sIGFuZCBvdGhlciBjaXRpZXMswqA8YSBocmVmPSJodHRwczovL3d3dy5nb3YudWsvZ292ZXJubWVudC9wdWJsaWNhdGlvbnMvYXNzZXNzbWVudC1hbmQtcHJvY3VyZW1lbnQtb2YtY29yb25hdmlydXMtY292aWQtMTktdGVzdHMvbGF0ZXJhbC1mbG93LWRldmljZXMtcmVzdWx0cyN0ZXN0LTMtY29yb25hdmlydXMtYW50aWdlbi1yYXBpZC10ZXN0IiB0YXJnZXQ9Il9ibGFuayIgcmVsPSJub29wZW5lciI-U2VlIHd3dy5nb3YudWsgZm9yIG1vcmUgaW5mb3JtYXRpb248L2E-ICh0ZXN0IDEgb24gdGhlIGxpc3QpLjwvbGk-DQo8bGk-PHN0cm9uZz5DRSBNYXJrZWQ6wqA8L3N0cm9uZz5Gb3IgcHJvZmVzc2lvbmFsIHVzZTwvbGk-DQo8bGk-PHN0cm9uZz5JU08xMzQ4NTo8L3N0cm9uZz4gSW5ub3ZhIG1hbnVmYWN0dXJlcyBpbiBhY2NvcmRhbmNlIHdpdGggdGhlaXIgSVNPMTM0ODUgUXVhbGl0eSBNYW5hZ2VtZW50IGFjY3JlZGl0YXRpb24uwqA8L2xpPg0KPC91bD4NCjwvZGl2Pg0KPC9zZWN0aW9uPg0KPC9kaXY-DQo8L2Rpdj4ifX0,/key/ddddc4968236b55a9d762a2eb80e021f851fb2339a4b4c292f6d95382b4760eb/
Images