Your trusted medical equipment partner

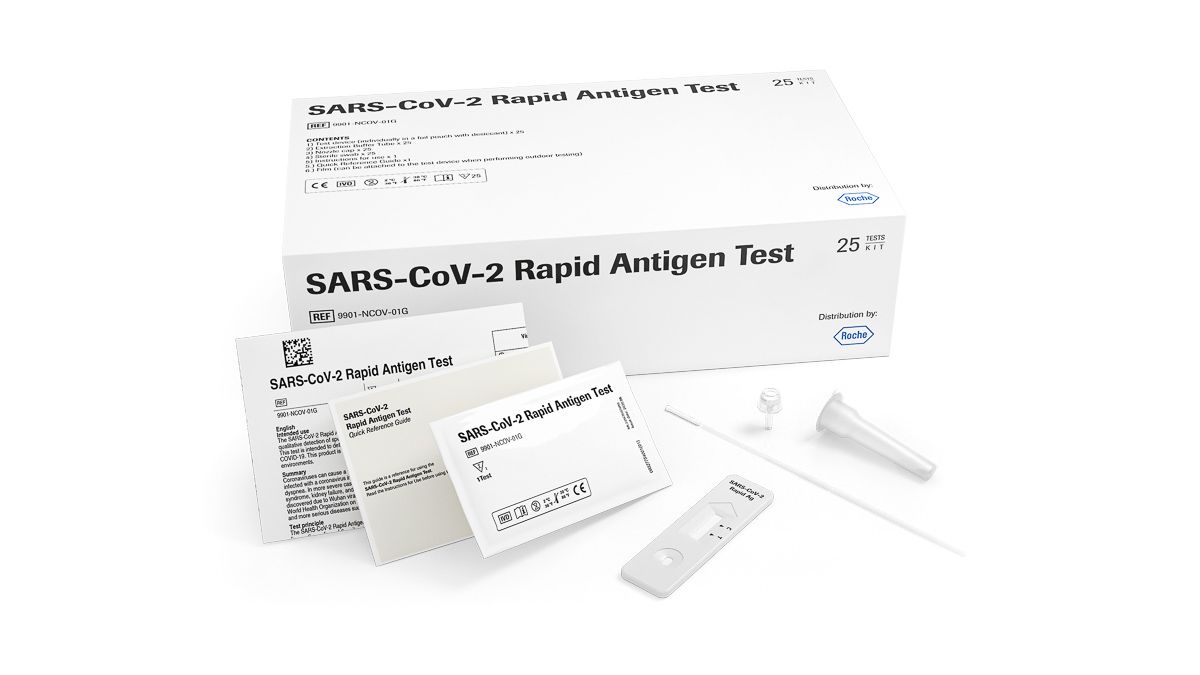
Roche SARS‐CoV‐2 (COVID-19) Rapid Diagnostic Antigen Test x25
Same day delivery is available for London and the South East. Please email sales@primarycaresupplies.co.uk for a quote.
This Roche Antigen testing kit facilitates the detection of COVID-19 with rapid return of results.
The SARS-CoV-2 (COVID-19) Rapid Antigen Test enables fast decision making e.g. whether patients need to be put in quarantine, reducing the risk of further spreading. In addition to that it allows for screening of individuals after confirmed exposure to a SARS-CoV-2 (COVID-19) infected person or individuals at risk of exposure such as healthcare workers.
Benefits
- Getting a quick result within 15-30 minutes – no need for a follow-up appointment to discuss the result
- Easy handling which does not require specific training
- No instrument required
- Allowing decentralized testing or access to testing in areas where laboratory testing is not available
Each kit contains 25 individually packaged, ready to use tests containing all equipment needed to perform a test, and comprises of:
- Test device – individual foil wrapped with desiccant
- Extraction buffer tube
- Sterile swab and Nozzle cap
- Film (to attach to test device to facilitate outdoor testing)
- Instructions for use and Quick Reference Guide
Features
- Five simple steps, with results after 15 minutes
- Quick and Easy to read result
- Excellent performance, Sensitivity 96.52% (Ct Value ≤ 30) and Specificity 99.2%
- CE Marked and MHRA approved test kits
Sensitivity: the ability of a test to correctly identify patients with a disease
Specificity: the ability of a test to correctly identify people without the disease
This product is strictly intended for professional use.
UK Government Tested
This product has been evaluated by the Government against the new Covid-19 variant summarising that the Roche test detects the new variant containing four amino acid changes from the original viral sequence.
External Clinical Evaluations
The main objective of the below presentation is to summarize key publications that deal with real world performance of the SARS-CoV-2 Rapid Antigen Test.
Additionally, factors that influence assay performance are described.
This presentation will be updated regularly.
SARS-CoV-2 Rapid Antigen Assay Clinical Performance
Summary of Conclusions:
- 14 studies presented with over 9’300 patient samples
- The sensitivity of the Roche / SD Biosensor POC Antigen assay was between 96.2 to 100% with a CT that is
considered to be associated with culture positive results. * - If the specimens are obtained ≤7 days after symptom onset for use with the Rapid Antigen test, it can help to
filter out the infected persons and prevent spread to the others. - First real world performance data confirms the primary use case for POC assay, however, more and larger
studies are needed
*The data from Uganda are not considered due to great discrepancy of the Ct values and categorization compared to all other republications.
This item line is exempt from our usual returns policy. This item is non-returnable or exchangeable.